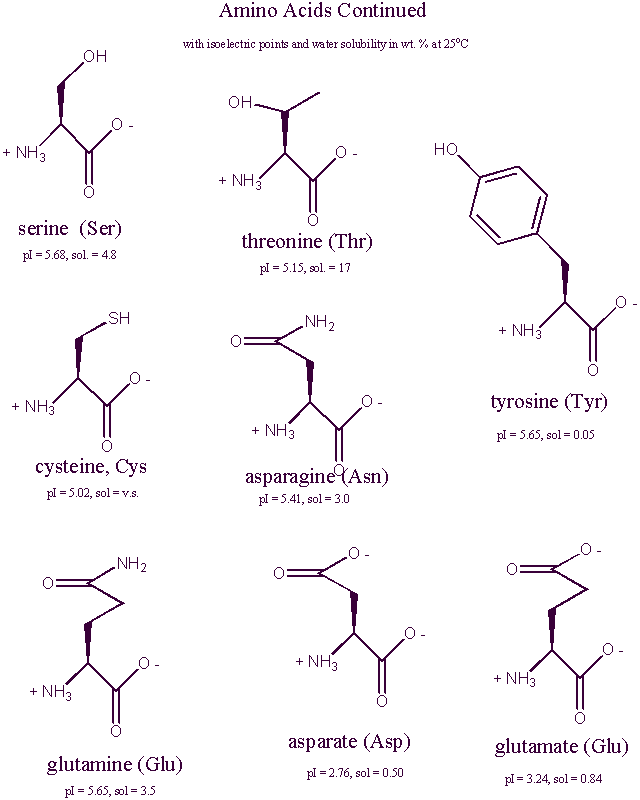
Amino Acid Structure At Physiological Ph . We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. 26.1 structures of amino acids. An amino acid has this ability because at a certain ph value (different. Each amino acid has its own pi value based on the properties of the amino acid. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a proton, becoming positively. At ph values above or below the. The structure of an amino acid allows it to act as both an acid and a base.
from www.drnerz.com
The structure of an amino acid allows it to act as both an acid and a base. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. At ph values above or below the. The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a proton, becoming positively. An amino acid has this ability because at a certain ph value (different. 26.1 structures of amino acids. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. Each amino acid has its own pi value based on the properties of the amino acid. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal.
Amino Acid Structures at Physiological pH
Amino Acid Structure At Physiological Ph The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a proton, becoming positively. The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a proton, becoming positively. At ph values above or below the. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. 26.1 structures of amino acids. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. Each amino acid has its own pi value based on the properties of the amino acid. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. The structure of an amino acid allows it to act as both an acid and a base. An amino acid has this ability because at a certain ph value (different. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins.
From pinbezy.weebly.com
Positively charged amino acids pinbezy Amino Acid Structure At Physiological Ph An amino acid has this ability because at a certain ph value (different. 26.1 structures of amino acids. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. At ph values above or below. Amino Acid Structure At Physiological Ph.
From www.chegg.com
Solved Knowing the information found in the table below, Amino Acid Structure At Physiological Ph 26.1 structures of amino acids. The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a proton, becoming positively. Each amino acid has its own pi value based on the properties of the amino acid. The structure of an amino acid allows it to act as both. Amino Acid Structure At Physiological Ph.
From mungfali.com
Amino Acid Structure Chart Amino Acid Structure At Physiological Ph The structure of an amino acid allows it to act as both an acid and a base. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. There are 22 amino acids that are found in proteins and. Amino Acid Structure At Physiological Ph.
From www.numerade.com
SOLVED Draw the amino acid Proline (Pro) as it would appear at pH 1 Amino Acid Structure At Physiological Ph The structure of an amino acid allows it to act as both an acid and a base. At ph values above or below the. An amino acid has this ability because at a certain ph value (different. Each amino acid has its own pi value based on the properties of the amino acid. We saw in section 20.3 and section. Amino Acid Structure At Physiological Ph.
From www.numerade.com
SOLVED Draw the predominant form the tyrosine amino acids at Amino Acid Structure At Physiological Ph The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a proton, becoming positively. The structure of an amino acid allows it to act as both an acid and a base. There are 22 amino acids that are found in proteins and of these, only 20 are. Amino Acid Structure At Physiological Ph.
From www.homeworklib.com
Draw the structure of glutamic acid at physiological pH, 7.4 HomeworkLib Amino Acid Structure At Physiological Ph There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. The structure of an amino acid allows. Amino Acid Structure At Physiological Ph.
From www.drnerz.com
Amino Acid Structures at Physiological pH Amino Acid Structure At Physiological Ph Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. An amino acid has this ability because at a certain ph value (different. The structure of an amino acid allows it to act as both an acid and a base. There are 22 amino acids that are found in proteins and of these, only. Amino Acid Structure At Physiological Ph.
From www.scribd.com
AMINO ACIDS AT PHYSIOLOGICAL PH PDF Amino Acid Structure At Physiological Ph The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. An amino acid has this ability because at a. Amino Acid Structure At Physiological Ph.
From edithghausero.blob.core.windows.net
The True Statement About Solutions Of Amino Acids At Physiological Ph Amino Acid Structure At Physiological Ph The structure of an amino acid allows it to act as both an acid and a base. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. Each amino acid has its own pi value based on the properties of the amino acid. The amine group of an amino acid has a relatively. Amino Acid Structure At Physiological Ph.
From quizlet.com
Modify lysine, below, to show the predominant form at pH 7. Quizlet Amino Acid Structure At Physiological Ph We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. 26.1 structures of amino acids. An amino acid has this ability because at a certain ph value (different. Each amino acid has its own pi value based on the properties of the amino acid. The zwitterion of an amino acid exists at a. Amino Acid Structure At Physiological Ph.
From basicmedicalkey.com
Structure, Nomenclature, and Properties of Proteins and Amino Acids Amino Acid Structure At Physiological Ph The structure of an amino acid allows it to act as both an acid and a base. An amino acid has this ability because at a certain ph value (different. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. Each amino acid has its own pi value based on. Amino Acid Structure At Physiological Ph.
From mungfali.com
Amino Acid Fischer Projection Amino Acid Structure At Physiological Ph 26.1 structures of amino acids. At ph values above or below the. An amino acid has this ability because at a certain ph value (different. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and. Amino Acid Structure At Physiological Ph.
From www.redbubble.com
"20 Amino Acids Physiological Structure Version" by Compound Interest Amino Acid Structure At Physiological Ph Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a. Amino Acid Structure At Physiological Ph.
From www.chegg.com
Solved Given the amino acid at pH = 7.4, identify the amino Amino Acid Structure At Physiological Ph The amine group of an amino acid has a relatively high pka, so at physiological ph (about 7), it will tend to bind a proton, becoming positively. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Each amino acid has its own pi value based on the properties of the amino acid. An amino. Amino Acid Structure At Physiological Ph.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and Amino Acid Structure At Physiological Ph The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Each amino acid has its own pi value based on the properties of the amino acid. An amino acid has this ability because at a certain ph value (different. The amine group of an amino acid has a relatively high pka, so at physiological ph. Amino Acid Structure At Physiological Ph.
From aminodotnet.weebly.com
Nature of Amino Acids Amino Acid Structure At Physiological Ph 26.1 structures of amino acids. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. The structure of an amino acid allows it to act as both an acid and a base. Each amino acid has its own pi value based on the properties of the amino acid. At ph values above or below. Amino Acid Structure At Physiological Ph.
From courses.lumenlearning.com
Amino Acids Biology for Majors I Amino Acid Structure At Physiological Ph An amino acid has this ability because at a certain ph value (different. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Each amino acid has its own pi value based on the properties of the amino acid. 26.1 structures of amino acids. At ph values above or below the. The structure of an. Amino Acid Structure At Physiological Ph.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Amino Acid Structure At Physiological Ph Each amino acid has its own pi value based on the properties of the amino acid. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal. At ph values above or below the. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. The structure. Amino Acid Structure At Physiological Ph.